Element: Francium (Fr)
Atomic Number: 87
Mass: Numerous isotopes ranging in mass from 199 amu to 232 amu, none of them stable. The only ones laser cooled are the five between 208 amu and 212 amu, plus the one at 221 amu.
Laser cooling wavelength: 718 nm
Doppler cooling limit: 182 μK.
Chemical classification: Alkali metal, column I in the periodic table. The heaviest known alkali, it’s presumably metallic in appearance, if you could ever get enough of it together to look at.
Other properties of interest: Francium has no stable isotopes, and the longest lifetime of any of its isotopes is around 20 minutes, for francium-221. Francium-210, the isotope with the most extensive laser cooling experiments, has a half-life of about 3 minutes.
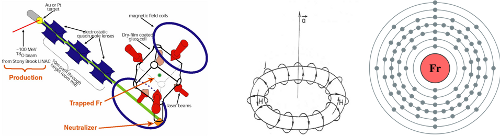
History: The “no stable isotopes” thing would seem to make francium an unlikely candidate for laser cooling, but it’s of significant interest to people looking for parity non-conservation (PNC) in atomic systems. Parity is one of the fundamental symmetries physicists like to talk about, corresponding roughly to a mirror reflection. It’s known to not be perfectly conserved, though the combination of charge, parity, and time-reversal symmetry (CPT) should be. CP symmetry violation is related to the matter-antimatter imbalance in the universe, so measurements of just how much violation of these symmetries exists in nature has important implications for fundamental physics. PNC experiments concentrate on heavy atoms, because the relevant effects get larger as the nuclear charge increases (of particular interest is the “anapole moment,” a cartoon of which is in the middle of the image up top), and some of the best measurements to date have been in cesium (which will get its own trading card eventually); the PNC effects in francium are expected to be an order of magnitude larger, so it’s worth some effort to trap.
Francium-221 is produced in the thorium decay chain, so it was trapped and cooled in Boulder using a fairly standard oven source supplied by the decay of unstable isotopes embedded in a platinum ribbon. The more impressive operation was undertaken at Stony Brook, where they made francium by directing a high-energy beam of oxygen ions onto a gold surface. This produced francium ions which were extracted from the gold by heating the target, accelerated some distance away from the target, neutralized by contact with a yttrium foil, and then passed into a vapor cell where the laser cooling and trapping took place. A schematic of this is the left-hand component of the “featured image” for this trading card. Amazingly, given the number of things that had to go just right, this works a treat, and they caught a few thousand atoms at a time in the mid-1990’s, increasing to a hundred-odd thousand in the second generation.
The original francium experiment was shut down because the accelerator at Stony Brook was closed. Groups in Italy and Japan have built their own systems, though, and a lot of the original francium team (including Seth Aubin who was a grad student at Stony Brook and is not a professor at William and Mary with a nice web page that I stole these graphics from) have re-assembled to make a new apparatus using the accelerator at TRIUMF in Canada. I wrote up a visit to Luis Orozco’s lab at Maryland where a lot of stuff was being put together back in 2008; they’re up and running now.
Random fun things: In talks, the Stony Brook crowd used to note that at any given instant there is probably something like a gram of francium on the entire Earth.
Art: The cartoon version of francium is a melting Terminator, but alas the Comic Book Periodic Table only includes a passing mention that isn’t even spelled correctly.
Francium-221 is produced in the thorium decay chain
Is there some new physics of which I was previously unaware? There are four decay chains, one for each of the possible values mod 4 of the mass number. Three of these start from isotopes with half-lives of hundreds of millions of years or longer: Th-232 (0 mod 4), U-238 (2 mod 4), and U-235 (3 mod 4). 221 is 1 mod 4, so it’s part of the other decay chain, in which the longest lived isotope is probably U-233. You can make U-233 by getting Th-232 to absorb a neutron (two beta decays follow), but the neutron has to be of the right energy, because Th-232 is fissile by neutrons in a different energy range. However, that’s not what I would think of as the “thorium chain”.
I’m tempted to claim that a sa deliberate error to see if anyone is paying attention to these, but really, it’s just a mistake. I knew it was produced by the decay of something or another, but grabbed the wrong radioactive element from memory, and failed to check it.