The last post in this series on the core technologies of cold-atom physics dealt with optical molasses, where you use the scattering of light to exert forces on atoms to make them very, very cold. It turns out, they end up even colder than the simple theory would lead you to expect, which is very surprising, but also essential to the revolutionary impact of cold atom physics. If you were stuck with the Doppler cooling limit temperatures, laser cooling probably wouldn’t be as big a deal as it is now.
You can do better, though, thanks to the interaction of several bits of physics that go beyond the simplest model you would think about. And one of the keye elements is the ability to use light to exert forces on atoms without scattering photons.
Yeah, you said that at the end of the last post, and it didn’t make any sense. I thought the whole game here was based on transferring momentum from light to atoms. How do you do that without scattering photons? Well, there are some subtleties to the interaction between light and atoms, and the language we use to talk about them. There’s a sense in which this is a bit of a semantic dodge, in that it turns on a particular technical meaning of scattering, where a photon comes in, gets absorbed, and then gets re-emitted through spontaneous emission in some random direction.
Yeah, but isn’t that what happens when light interacts with atoms? It’s one thing that can happen as part of the interaction, but not the whole story. There’s also stimulated emission, which provides the “s” in the acronym that became the word “laser”: “Light Amplification by Stimulated Emission of Radiation.” Stimulated emission involves an atom that gets excited and then de-excited by interaction with a light field. The photon that comes out is indistinguishable from the photon that stimulated it. That’s what makes a laser– if you shine light through a sample of excited atoms, the light you put in makes them release new photons just like the ones you sent in, massively amplifying your original light.
And this is the thing that exerts forces without scattering. Yes and no. I mostly mention it as an example of how the interaction between light and atoms is more complicated than just “atom absorbs photon, atom emits photon.” What really matters here is the mixing of states.
Okay, what? So, the situation we’re generally interested in involves an atom with two states, right? A ground state, that’s the lowest energy possible in the atom, and an excited state that’s somewhat higher. When an atom interacts with light, you can excite it from the ground state to thee excited state, which we learn in grade school involves absorbing a photon.
If you dig into the details, though, there’s more to it than that. See, those energy states in the atom are for a particular set of interactions, including only the nucleus and its electrons. When you add the light field, you modify that system, and if you’re going to do things right, you need to re-calculate the allowed energy states to include the presence of a light field with a particular frequency and intensity (or a particular number of photons at a particular frequency).
That sounds like a huge hassle, having to re-do the system all the time. It could be, but the nice thing about quantum mechanics is that once you have the allowed states for one problem, you can write the answer to absolutely any other problem in terms of those states. So, what we do is to solve the easy problem of an atom all by itself, and then think about the states of the atom interacting with the light as combinations of the states of the atom by itself.
For our two-level atom, this means that when the light is on, the two states are really a mixture of the two “bare” states. You have a low-energy state that is mostly ground state, plus a bit of excited state, and a high-energy state that is mostly excited state, plus a bit of ground state. Provided a few fairly easy to meet conditions hold, these combinations are the proper thing to think about.
And this helps you… how? Well, the energies of the two combined states aren’t quite the same as the energies of the two original states. The energy of the low-energy state goes up or down by a small amount that depends on the frequency and intensity of the laser. And that gives you a new way t push atoms around, because if you’ll forgive a little anthropomorphization, atoms always want to be in the place where their energy is lowest.
So, if you create a light field that varies in intensity over some region of space– say, by focusing a laser to a small point– you also create an energy landscape for the atoms that varies over that same region of space. If you pick your laser parameters correctly, the low-energy combined state will have its lowest energy at the center of the laser spot, and an atom off to the side of the laser will feel a force that pulls it toward the center.
And once it’s there, it absorbs photons, right? Actually, no. This “dipole force” exists even if the laser is far away from the frequency the atoms like to absorb. You can have a substantial dipole force without ever scattering a single photon, in the sense I mentioned above, where the atom ends up in the excited state, and then spontaneously decays to the ground state emitting a photon in a random direction.
How does that work? Well, when you go through the math, the probability of finding the atom in the excited state depends on the intensity of the laser divided by the square of the “detuning,” the difference between the laser frequency and the natural frequency the atom wants to absorb. The energy shift, on the other hand, depends on the intensity divided by just the detuning. If the detuning is big, the excited-state probability will always be smaller than the energy shift, so if you have enough laser power, you can get a large energy shift while keeping the probability of excitation very small.
Define “big” and “enough.” Well, for scattering forces, you typically work with lasers that are within tens of megahertz of the natural frequency– within a few parts per billion, give or take– and intensities of a few milliwatts per square centimeter. For a dipole trap, you’ll often use lasers whose wavelength is a hundred nanometers or more away– a frequency difference of maybe 20%– and laser powers of a watt or more focused down into a spot a few tens of micrometers across.
That’s big, all right. Yep. And some of those lasers can be kind of scary to work with. But it’s worth it to be able to trap atoms without exciting them.
So, this dipole force business supersedes the optical molasses stuff? No, for two reasons. One is that the intensities required to get a substantial force require focusing a big laser down to a tiny spot, so they don’t have much of a capture range– that is, they can only affect atoms very, very close to the focus of the laser. Optical molasses, on the other hand, uses low intensities, and you can set up molasses beams with a diameter of a centimeter or more and cool atoms in a huge range of space.
The other problem is that in physics jargon, the dipole force is a “conservative” force– it arises from a change in the internal energy states of the atoms, and those states depend only on the position. This kind of force can’t dissipate energy– the total energy of the atoms stays the same. So an atom off to the side of the trap feels a force pulling it toward the center, but when it gets to the center it’s moving, and doesn’t stay there, but just cruises on by.
If you have a strong enough laser, you can confine a sample of atoms in this kind of trap, but it’s hard to get enough laser power to trap anything that isn’t already cold. A gas of atoms that already have significant kinetic energy due to their motion will just roll right through the trap, speeding up a bit but not being confined.
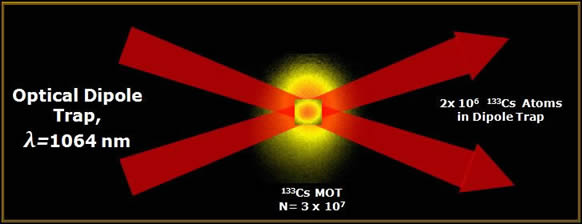
So, what’s the point? Well, if you combine a dipole force with optical molasses, you can load atoms into the trap by cooling them. The light scattering forces in optical molasses allow energy dissipation, so they can take atoms that are too hot to be caught by the dipole trap and cool them down to the point where they don’t have enough energy to get out of the focus of the laser. The figure up top shows a schematic of a “crossed beam” dipole trap at the University of Chicago, where two lasers focus at the same point in space, and they can load tens of millions of atoms into that trap.
If you still need the molasses, though, what’s the point? Well, dipole traps are very versatile. You can change the strength of the trap just by changing the intensity of the laser, or switch it off completely, and those are things you can do very fast with light. You can also move them around in space very easily by changing where you put the focus of your laser, so some groups use dipole traps to shift cold atoms from one part of a complicated system to another. And you can trap lots of different internal states in the same dipole trap, which isn’t true of the magnetic traps that we’ll talk about later. So, there are lots of reasons to want to load cold atoms into a dipole trap.
So, the idea is just to get a bunch of atoms cold, then trap them in the focus of a laser? That’s the main way people use these, and the simplest. You can also use this force to push atoms away from the focus of a beam, though, by using a different frequency– if you detune the beam to a lower frequency than the atoms want to absorb, they’re drawn into the light, but if you detune to a higher frequency, they’re pushed away from the light. That can also be used to make traps, by building “boxes” and the like, which can be useful for some kinds of experiments.
Okay. I still don’t see how to understand this in terms of photons, though. Well, in a very loose sense, you can think of the energy shift and the mixing of the ground and excited states as coming from the absorption and stimulated emission of photons. The atom is flipping back and forth between the two states very rapidly as a result, so quickly that if you look at it over a time much longer than the flipping, you see a smeared-out mix of the two, with a different energy. In that sort of picture– which is not that great, by the way– this does in fact result from atom-photon interactions, but since there’s never any spontaneous emission, we don’t see photons scattered in a direction other than the direction of the original laser. Which is why we don’t talk about it in terms of scattering– nothing has been “scattered” in the sense of changing direction.
(There are a handful of people who regularly read this blog who might want to bludgeon me with copies of Claude Cohen-Tannoudji’s quantum optics texts after reading that, by the way. This is not remotely a quantitatively accurate way to think about this process, but it’s the best I’ve got right now. I welcome better explanations in the comments.)
All right, I guess. But how does this get you extra cooling? Well, this is the second of four pieces of information that you need to understand sub-Doppler cooling, after the fact I mentioned in the last post, that the interaction between the different beams in the molasses creates an interference pattern. The other two pieces will come along in the next post.
Oh, fine. Any last notes on the dipole force and dipole force traps? Well, the only other thing that comes to mind is that these have been an unusually rich source for cutesy acronyms. The umbrella term that gets the most use is “FORT” for “Far Off Resonance Trap,” but specific implementations have had names like “UBOAT” (Ultra-Blue Optical Atom trap), “ROBOT” (ROtating Beam Optical Trap), and “RoDiO” (Rotating Dipole Optical (trap)). I don’t know what it is about dipole force traps that makes people go all goofy, but there you go.